What are Alkaline, Reverse Osmosis, and Ionized Waters?
Alkaline water has a pH above 7.0 and is relatively easy to come by these days. Some bottled waters are labeled and marketed as alkaline. Some of these are bottled from a naturally alkaline spring source but that doesn’t mean it’s 100% alkaline spring water. The bottled water industry is not regulated and the water does not have to be tested. Not only that, but bottled water labeled and sold as spring water is only required to have 20% of the water be spring water. Alkalinity can be created by adding chemicals such as baking soda (sodium bicarbonate), lye (as is done in many municipal water supplies to bring the pH to neutral), and even potassium cyanide. So, obviously, testing alkalinity in and of itself is not necessarily the best way to determine if a water is good for you or not.Reverse osmosis (RO) is a process that removes all the impurities from water. It eliminates the nasty chemicals such as chlorine, ammonia, fluoride, etc. and heavy metals which we don’t want to consume AND also removes the alkalizing minerals naturally present in water that our bodies need for optimum health. Most all water labeled as “pure” has been through a reverse osmosis or distillation process. RO water is very acidic unless alkalizing minerals have been added back into it afterwards.
Ionized water has been run through an electrolysis chamber in which electrodes separate the positive and negative ions present in [by means of the mineral content of] the water. Two separate streams of water exit the chamber, one alkaline (referred to in the diagram below as Kangen) and the other acidic. The alkaline water created through electrolysis is primarily hydroxyl (OH–) atoms and the acidic water created is primarily hydrogen (H+) atoms.
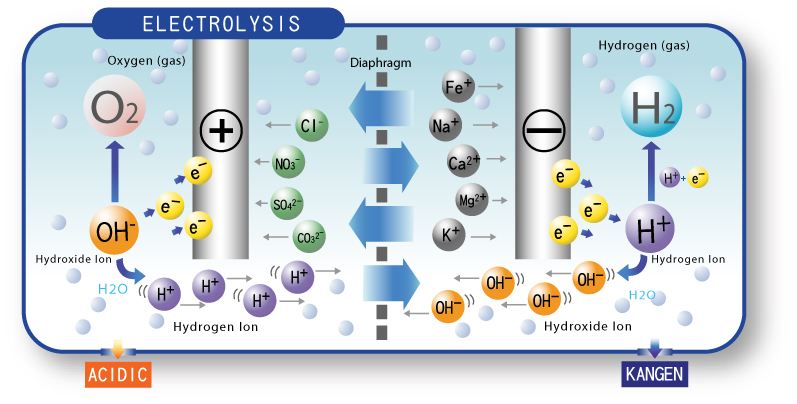
The negative charge of the hydroxyl atoms found in ionized water act as antioxidants and neutralize the positively charged free radical atoms which do damage to the body and create the decay associated with aging. Not all alkaline water contains these negative ions and some may even have positive ions — the oxidizing free radical atoms.
